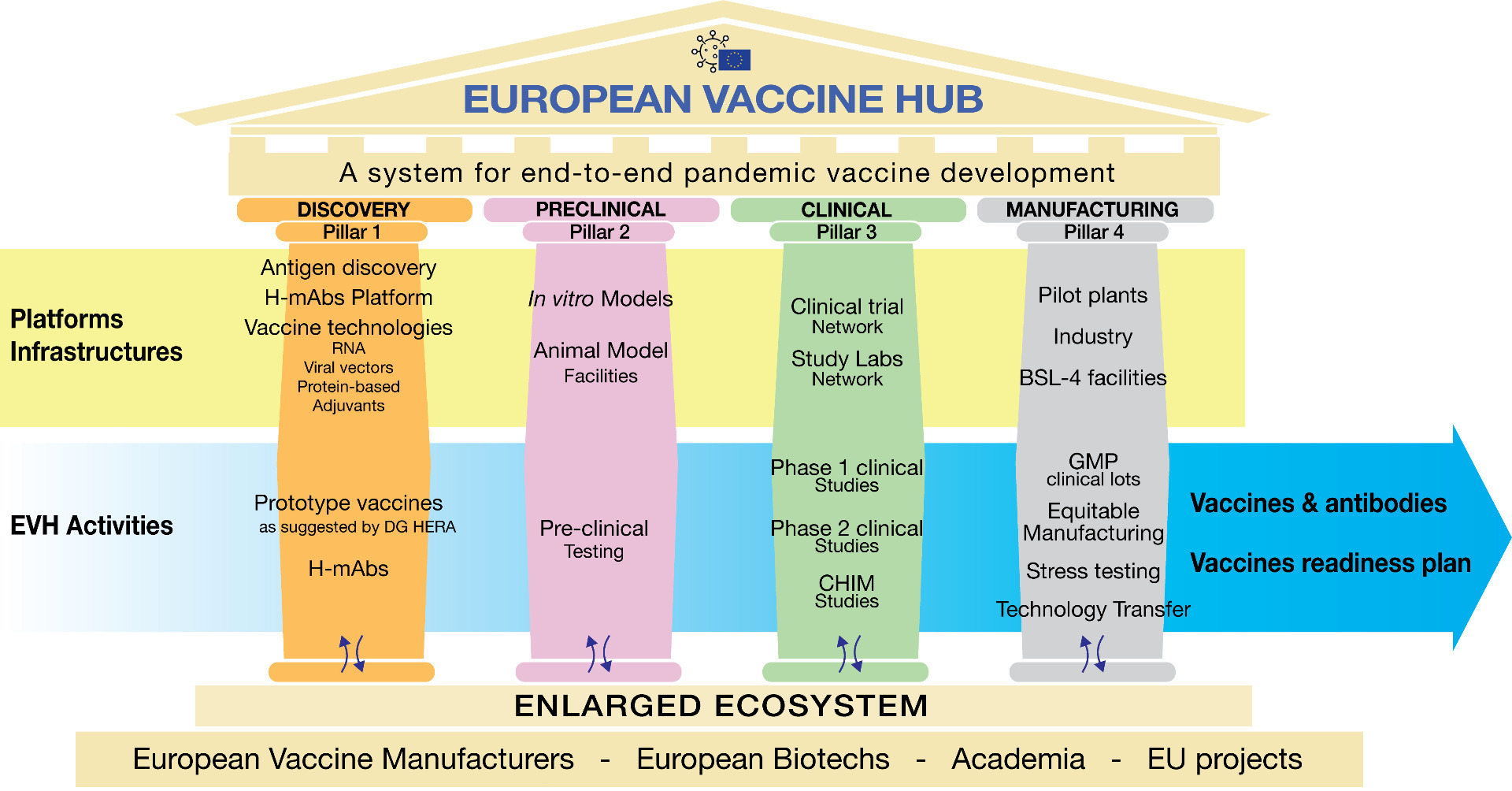
Main objectives
- Develop and implement a pandemic vaccines readiness plan for Europe
- Develop pandemic vaccine candidates for agreed pathogens up to Phase 2 clinical trials, including through relevant in vitro and in vivo models.
- Organise and optimise the clinical evaluation of candidate vaccines including in Phases I and II and the appropriate and supportive use of controlled infection facilities to speed up clinical development.
- Ensure rapid delivery of vaccine candidates for trial and mass production to manufacturing partners
- Set up shared, ready-to-use and scalable state-of-the-art technology platforms covering all phases of vaccine development with a focus on high-yield and rapidly adaptable production systems.
- Prepare and support technology transfers among EU partners and other eligible entities as part of the creation of a centre of shared knowledge for pandemic-relevant vaccine development.
- Prepare and perform stress tests of pandemic vaccine production, including on critical supply-chain element
- Prepare relevant master clinical trial protocols combining its activities to reach out to clinical trial capacities/networks and at scale production initiatives at national and European levels
- Implement a pandemic planning platform operating in the most cost-effective way