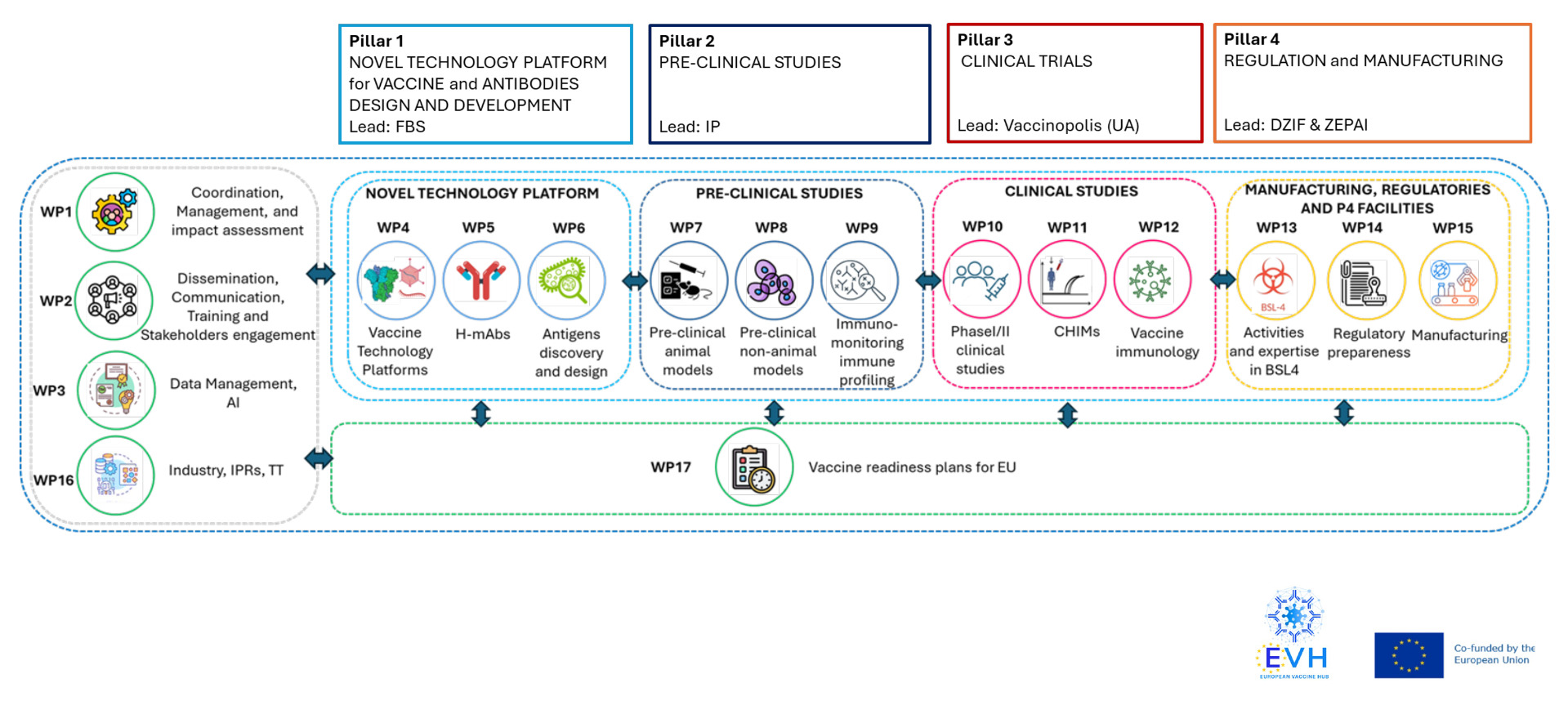
Structure
EVH is organised around 4 Pillars, each supporting key activities and infrastructures of the vaccine development pipeline:
- Pillar 1 – Discovery, led by Fondazione Biotecnopolo di Siena (FBS, Italy)
- Pillar 2 – Pre-clinical Studies, led by Institut Pasteur (IP, France)
- Pillar 3 – Clinical Studies, led by Vaccinopolis (UA, Belgium)
- Pillar 4 – Regulation & Manufacturing, led by Deutsches Zentrum für Infektionsforschung (DZIF, Germany) and Zentrum für Pandemie-Impfstoffe und -Therapeutika (ZEPAI, Germany)
In the event of pandemic, each pillar will set up and implement the platforms necessary for rapid vaccine development. EVH will engage and reinforce the complete ecosystem of European industry and academia, promoting collaborations and the exchange of know-how and projects.
Each Pillar is structured into thematic Work Packages (WPs). In addition to Pillar-specific WPs, EVH includes additional WPs that support cross-cutting activities and reinforce the integrated, end-to-end approach to pandemic vaccine development and preparedness.